Abstract
Background: The combination of hyperfractionated cyclophosphamide, dexamethasone, vincristine, and doxorubicin, alternating with high-dose methotrexate (MTX) and cytarabine (HyperCVAD) constitutes one of the standard regimens for the treatment of acute lymphoblastic leukemia (ALL). In this treatment scheme, MTX is administered at a dose of 1000 mg/m2 over 24 hours, along with supportive care including leucovorin rescue until MTX clearance is achieved (serum MTX level < 0.1 µmol/L). Mini-HyperCVD was designed to mimic HyperCVAD but with the omission of doxorubicin and significant dose reductions to minimize toxicity for elderly and relapsed or refractory patients. In this regimen, MTX is administered at a dose of 250 mg/m2 over 24 hours. Currently, leucovorin is also used to mitigate MTX toxicity as in the standard Hyper-CVAD regimen, but it may not be needed due to the significant dose reduction in MTX. MTX clearance and the need for leucovorin rescue in the mini-HyperCVD regimen has not been previously investigated.
Methods: The clearance of MTX in patients treated with the mini-HyperCVD (NCT01371630) and standard HyperCVAD regimens from 1/1/2010 through 3/31/2018 was retrospectively reviewed. MTX levels drawn at the conclusion of administration (0 hour level), 24 hours after the conclusion of MTX (24 hour level), 48 hours after the conclusion of MTX (48 hour level), and 72 hours after the conclusion of MTX (72 hour level) were collected. MTX clearance was defined as serum MTX level < 0.1 µmol/L. Descriptive statistics and student t-test comparing MTX levels between mini-HyperCVD and HyperCVAD regimens were performed. All patients with documentation of at least 2 serum MTX levels following MTX administration were included except for those with empiric MTX dosage reductions.
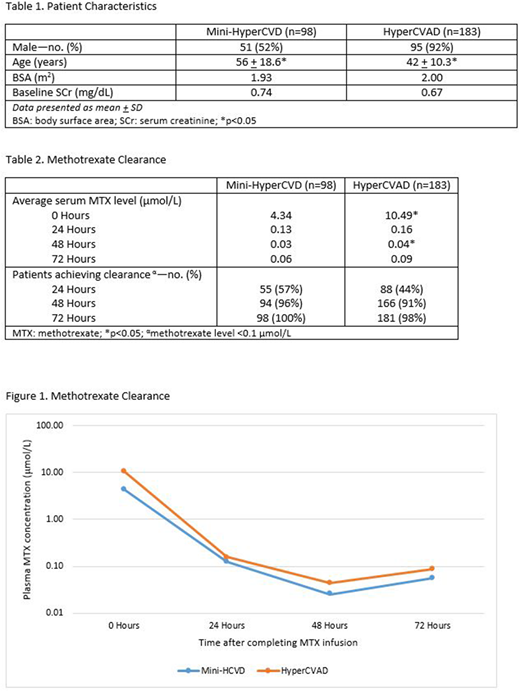
Results: Two hundred eighty-one MTX administrations as part of the mini-HyperCVD (n=98) or HyperCVAD regimen (n=183) at dosages of 250 mg/m2 or 1000 mg/m2 respectively, were included. Patients who received mini-HyperCVD were significantly older than those who received HyperCVAD (56 vs 42 years, p<0.05). Other patient characteristics including gender, body surface area (BSA), and baseline serum creatinine (SCr) were similar between the 2 cohorts (Table 1). The average serum 0 hour MTX level after mini-HyperCVD was significantly lower than after HyperCVAD (4.34 µmol/L and 10.49 µmol/L, respectively; p<0.05). Mean serum levels obtained at the 24, 48, and 72 hour time points were similar between treatment groups (Table 2; Figure 1). There were no significant differences in the proportion of patients who achieved clearance at 24, 48 and 72 hours post-MTX administration. At 24 hours after the completion of MTX administration, 55 of the 98 patients (57%) treated with mini-HyperCVD achieved MTX clearance and 88 of the 183 patients (44%) treated with standard HyperCVAD achieved clearance. Regardless of MTX dose, nearly all patients achieved clearance 48 hours after the completion of the infusion (mini-HyperCVD: 96%; HyperCVAD: 91%).
Conclusion: The peak serum methotrexate level observed with the mini-HyperCVD regimen is significantly lower than that observed with standard HyperCVAD due to the reduced MTX dosage of 250 mg/m2 compared to the HyperCVAD dose of 1000 mg/m2. Despite this dosage reduction, most patients still required 48 hours to achieve serum MTX level <0.1 µmol/L. Therefore to maintain optimal patient safety, MTX administration as part of the mini-HyperCVD regimen should continue to be administered with full supportive care including leucovorin rescue.
Ravandi:Orsenix: Honoraria; Xencor: Research Funding; Seattle Genetics: Research Funding; Jazz: Honoraria; Abbvie: Research Funding; Sunesis: Honoraria; Bristol-Myers Squibb: Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Abbvie: Research Funding; Amgen: Honoraria, Research Funding, Speakers Bureau; Seattle Genetics: Research Funding; Bristol-Myers Squibb: Research Funding; Sunesis: Honoraria; Astellas Pharmaceuticals: Consultancy, Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Jazz: Honoraria; Macrogenix: Honoraria, Research Funding; Orsenix: Honoraria; Macrogenix: Honoraria, Research Funding; Xencor: Research Funding. Short:Takeda Oncology: Consultancy. Sasaki:Otsuka Pharmaceutical: Honoraria. Thompson:Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Research Funding; AbbVie: Honoraria, Research Funding; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Honoraria, Membership on an entity's Board of Directors or advisory committees. Jain:Genentech: Research Funding; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Abbvie: Research Funding; BMS: Research Funding; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding; Infinity: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Research Funding; Pharmacyclics: Research Funding; Pfizer: Research Funding; Abbvie: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; ADC Therapeutics: Research Funding; Celgene: Research Funding; ADC Therapeutics: Research Funding; Adaptive Biotechnologioes: Research Funding; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologioes: Research Funding; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Verastem: Research Funding; Incyte: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding; Seattle Genetics: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cellectis: Research Funding; Astra Zeneca: Research Funding; Pfizer: Research Funding; Astra Zeneca: Research Funding; Servier: Research Funding; Infinity: Research Funding; Cellectis: Research Funding; Pharmacyclics: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. O'Brien:Aptose Biosciences Inc.: Consultancy; Alexion: Consultancy; Janssen: Consultancy; Gilead: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Kite Pharma: Research Funding; Pfizer: Consultancy, Research Funding; GlaxoSmithKline: Consultancy; Regeneron: Research Funding; Abbvie: Consultancy; Amgen: Consultancy; Celgene: Consultancy; Astellas: Consultancy; Pharmacyclics: Consultancy, Research Funding; Acerta: Research Funding; TG Therapeutics: Consultancy, Research Funding; Vaniam Group LLC: Consultancy. Jabbour:Abbvie: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Novartis: Research Funding; Pfizer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal